IO from SCIAMACHY measurements
Introduction Retrieval Data References Links Contact
 Introduction:
Introduction:
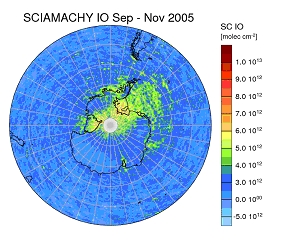
Fig. 1: IO slant columns on the Southern Hemisphere averaged over 3 months (Sep-Nov 2005). Highest values are found in the Weddell sea and the shelf ice areas.
Halogens (chlorine, bromine, iodine,…) play an important role in our atmosphere in different respects. Well known is the effect of chlorine species in the stratosphere, where they are responsible for the formation of the ozone hole. But also in the lower layers, in the troposphere, halogens lead to ozone destruction. Especially in the polar springtime periods, bromine species have been identified as a cause for local ozone depletion events, where nearly all ground-level ozone can be destroyed within short time. In addition to chlorine and bromine, also iodine influences atmospheric chemistry. It is very effective in ozone destruction and is additionally involved in the formation of fine particles in cases of high concentrations of iodine oxides. It is possible to detect iodine monoxide (IO) by means of the DOAS (Differential Optical Absorption Spectroscopy) technique. This has been done before using ground-based and balloon-borne instruments. Here, measurements of IO from the SCIAMACHY satellite instrument are discussed.
The Antarctic is an interesting place considering halogen chemistry. Here, the appearance of extended bromine oxide (BrO) clouds has been observed from satellite, and occasions with extremely low ozone concentrations close to the ground have been reported. Many open questions are still connected to the subject of halogen chemistry, one of them being the importance of iodine for ozone depletion and particle formation. In addition, the influence of iodine species on triggering and enhancement of bromine release is not yet fully understood. Certain chemical reactions in the liquid phase, for example on aerosol surfaces, can amplify the activation of gaseous bromine, and also cross reactions between IO and BrO can lead to additional release of atomic bromine and iodine, thereby increasing the chain lengths of the catalytic cycles.
Although the IO absorption signal is very small, it can be retrieved from satellite measurements of backscattered solar light using the DOAS method. As an example, the observed IO distribution for September to November 2005 is shown in Fig. 1. More on the retrieval and the detection limit can be found below.
The seasonal cycle extracted from the satellite data for Halley Station (including all measurements within a square of 500 km side length enclosing Halley Station) is shown in Fig. 2. A maximum of IO slant columns with values around 7-8x1012molec/cm2 is observed in Antarctic springtime (around October), still positive but lower values during the summer period and a second slightly less pronounced maximum in autumn. During the winter, hardly any measurements from satellite are available for Halley Station due to darkness, but in the dataset the IO amounts decrease towards winter. High IO values are not expected for the winter season as sunlight would be needed for the photolysis of the precursor substances. The variation in IO columns is a product of the seasonal cycle of the precursor substances and available sunlight necessary for the photolysis. This seasonal cycle is repeated in each of the three analysed years from 2004 to 2006.
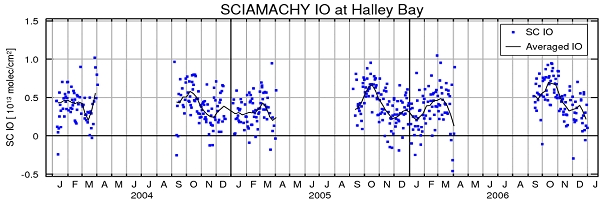
Fig 2: Seasonal variation of IO. The figure shows SCIAMACHY IO slant columns within 500 km of Halley Station for the three years from 2004-2006; daily values in blue and a two week average in black. This seasonal cycle matches well with ground based long path DOAS results, cp. (Saiz-Lopez et al., 2007).
 Retrieval and Detection Limits:
Retrieval and Detection Limits:
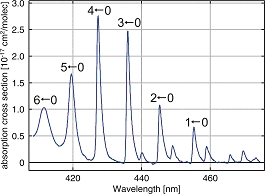
IO has very strong differential absorption structures in the blue wavelength region as can be seen in Fig. 3 below. The maximum absorption cross section, depending on the resolution of the spectrometer, lies around smax~2.8x10-17cm2/molec. Using a fitting window for the DOAS retrieval of 416–430 nm, two of the absorption peaks are included.
The detection limit of the instrument determines in which cases the IO can be retrieved. Apart from possible systematic effects or errors, this detection limit depends on the signal-to-noise ratio (S/N) of the satellite measurement. The S/N again depends on atmospheric and measurement related parameters such as integration time, the amount of spatial or temporal averaging, and ground spectral reflectance. This is again also dependant on the specific wavelength region under consideration. For the case of IO, the best detection can be expected over bright surfaces, such as the Antarctic for example.
For IO slant columns and a surface spectral reflectance of 90%, the detection limit is given by SClim= 7x1012molec/cm2 for a single measurement. For a surface spectral reflectance of 5% instead of 90%, the IO slant column detection limit for a single measurement corresponds to 2x1013molec/cm2. For 90% surface spectral reflectance and the spatially averaged ground scene of 60x120 km2, used in this work, the limit is reduced to 3x1012molec/cm2. In the monthly or even longer time average, the overall detection limit can further decrease.
In the retrieval of IO by the DOAS technique, other effects and absorptions have to be taken into account. The current fit includes several effects such as the absorption of NO2, O3, the Ring effect, and a quadratic polynomial accounting for the broad band structures in the spectrum (resulting from instrumental characteristics and elastic scattering). The results of the fitting procedure are the slant columns of the respective trace gas, which can be converted into vertical columns using appropriate airmass factors. As little is known on the vertical profile of IO, so far only slant columns are shown.
For more details on the retrieval and the results see the paper of Schönhardt et al., 2007.
 Data:
Data:
If you are interested in more SCIAMACHY IO data, please contact Anja Schönhardt.
 References:
References:
-
Schönhardt, A., Richter, A., Wittrock, F., Kirk, H., Oetjen, H., Roscoe, H. K. and Burrows, J. P., Observations of iodine monoxide (IO) columns from satellite, Atmos. Chem. Phys., 8, 637-653, 2008
- First observations of iodine oxide columns from satellite, A. Schönhardt et al., EGU meeting, April 2007
-
Saiz-Lopez, A., Mahajan, A. S., Salmon, R. A., Bauguitte, S. J.-B., Jones, A. E., Roscoe, H. K., and J. M. C. Plane: Boundary layer halogens in coastal Antarctica, Science, 317, 348, DOI:10.1126/science.1141408, 2007.
-
Spietz, P., Gomez Martin, J. C., and Burrows, J. P.: Spectroscopic studies of the I2/O3 photochemistry, Part 2: Improved Spectra of Iodine Oxides and Analysis of the IO Absorption Spectrum, J. Photochem. Photobiol. A, 176, 50-67, doi:10.1016/j.jphotochem.2005.08.023, 2005.
 Links:
Links:
-
More information on SCIAMACHY can be found here.
-
Many SCIAMACHY related links can be found on the German SCIAMACHY page.
-
More on SCIAMACHY BrO measurements can be found here.
-
For information on the satellite and other ENVISAT instruments check the ESA ENVISAT page
 Contact:
Contact:
If you are interested in more information or SCIAMACHY IO data, please contact Anja Schönhardt.